 |
MAGISKA MOLEKYLERS WIKI |
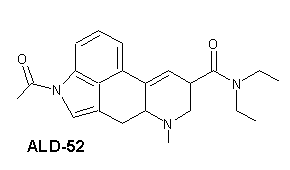
ALD-52
Namn: K├żnd i USA i slutet av 60-talet under namnet "Sunshine" eller "Orange Sunshine", l├żs mer nedan.
Generell information
ALD-52 ├żr en ergotalkaloid och LSD-analog med liknande potens och effekter. Men som Alexander Shulgin f├Črklarar i citatet nedan finns det ├żven en del subjektiva uppfattningar om skillnader:
| ŌĆ£ | This material has been explored in the 50-175 microgram range and there are a number of human trials reported, with varying conclusions. One found that there was less visual distortion than with LSD and it seems to produce less anxiety and was somewhat less potent than LSD. Another report claimed it was more effective in increasing blood pressure. Yet another could not tell them apart. ŌĆö TiHKAL av A. Shulgin[1] |
ŌĆØ |
ALD-52 p├źstods ha s├źlts i Kalifornien under namnet "Orange sunshine" i slutet av 1960-talet. En kemist stod ├źtalad f├Čr att ha tillverkat LSD f├Črs├Čkte anv├żnda ALD-52 som f├Črsvar i en r├żtteg├źng genom att h├żvda att kemisten tillverkat den lagliga drogen ALD-52 som fallit s├Čnder till LSD i polisens f├Črvar/provtagningsf├Črfarande. 2017 kom det fram att kemisten som ├źkte fast hela tiden hade tillverkat LSD och historien om ALD-52 var fabricerad[2]
Det finns uppgifter som g├Čr g├żllande att ALD-52 omvandlas till LSD i kroppen:
| ŌĆ£ | Monroe and Heath (1961) compared activity of several LSD analogs in monkeys and cats. LSD was the most active. DAM and LPD (see Table 6) produced moderate EEG activity and about 10% of LSD's psychological activity. MLD-4 and LSM had minimal effects on the EEG and 20-40% of LSD's psychological activity. l-LSD, BOL, and UML were inactive. In sharp contrast ALD-52 was as active psychologically as LSD but produced minimal EEG changes. This could be due to the slow release of LSD from its acetyl derivative by hydrolysis in the body. ŌĆö Hallucinogens (1967)[3] |
ŌĆØ |
LSD-analogen 1P-LSD har visat sig omvandlas till LSD i m├żnskligt blodserum.[4][5] Det b├Čr ge en h├Čg sannolikhet att ALD-52 genomg├źr samma hydrolys p├ź 1-positionen ├żven om det inte bevisats. Den k├żnde kemisten David Nichols h├źller med:
| ŌĆ£ | It is well known in organic chemistry that N-acyl indoles of all kinds hydrolyze easily. Whether or not it hydrolyzes in the body has not been tested. Taken orally, the low pH of the stomach would likely take it off readily. At some point, you have to accept that some mechanisms in organic chemistry are accepted, although the exact experiment appears not to have been done on ALD-52.
.... I donŌĆÖt think any mysticism is involved. It is a simple extrapolation from de-acetylation of all indoles under mild conditions, to deacetylation of ALD-52 in vivo. From what we now know about the structure-activity relationships of LSD analogues, the N(1)-acetyl compound would not be active. If it is active in man, it is only by hydrolysis of the acetyl group to give LSD. |
ŌĆØ |
Kemi
| Systematiskt namn | 1-Acetyl-N,N-diethyllysergamide | |
| Trivialnamn | ALD-52 | |
| Kemisk formel | C22H27N3O2 | |
| Sm├żltpunkt | ||
| Kokpunkt | ||
| Molmassa | 367,48 g/mol | |
| Densitet | 1.20 +/- 0.1 g/ml | |
| L├Čslighet | L├żs under extrahering |
Refraktivt index (uppskattat): 1.619 +/- 0.05
Ytsp├żnning (uppskattat): 43.0 +/- 7.0 dyne/cm
Externa l├żnkar
- Ōåæ TiHKAL #26. LSD-25
- Ōåæ Erowid: Tim Scully Describes Failed ALD-52 Legal Gambit
- Ōåæ The Hallucinogens (1967) Av Abram Hoffer & Humphry Osmond
- Ōåæ Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD) (Brandt, 2015)
- Ōåæ Pharmacokinetics and subjective effects of 1PŌĆÉLSD in humans after oral and intravenous administration (Grumann, 2020)
- Ōåæ Bluelight: The Big & Dandy 1P-LSD Thread, Volume 1
Magiska Molekyler: ALD-52, kontrollerad substans?, Hj├żlp mig reda ut det h├żr.
Magiska Molekyler: ALD-52, eller Min K├żre V├żn Orange Sunshine, Om LSD-analogen ALD-52.